Proteins in cells are typically composed of three animo acid chains, wrapped up tightly around each other. A single cell may contain up to 20 million copies of the same protein, whilst there are around 50,000 different proteins encoded in the human genome.
A computer-generated representation of a protein complex
(Courtesy of Lucy Colwell/www.pymol.org)
In the treatment of hair and blood vessels we are particularly interested in the melanin and haemoglobin proteins. Using heat generated by absorbed light energy, we can specifically target those proteins and induce irreversible denaturation – this is when the amino acid chains unravel, and the protein dies.
To achieve this, we need to raise the temperature of the proteins to some level for a certain time. As I have mentioned previously (my blog post), irreversible denaturation occurs when the ‘correct’ combination of temperature and time is achieved. The thing is, there are an infinite number of these combinations!!
We use the Arrhenius Damage Equation to calculate the required temperature/time combinations (we published a paper on this a few years ago).

The Arrhenius Damage Equation: ‘W’ represents the total amount of protein damaged by applying a temperature, T, for a time ‘t’. The variables ‘A’, ‘Ea’ and ‘R’ are all dependent on the individual proteins.
Using data collected in laboratory experiments on tissues, we can plug in some numbers into the above equation and calculate the temperature/time combinations which will give us our goal.
The easiest way to view these combinations is with a ‘denaturation triangle’.
The triangle clearly shows how the time required to achieve the goal of irreversible denaturation (when W = 1) shortens dramatically as the temperature increases.
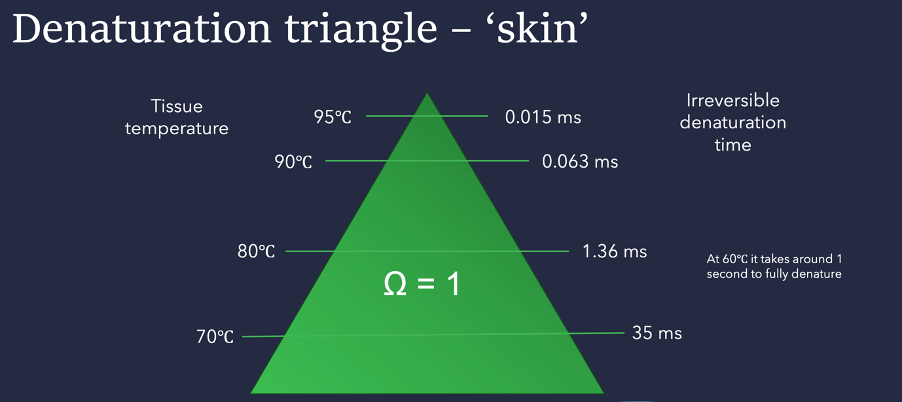
For “bulk skin” (which includes all the tissues found in the skin), the triangle shows that a temperature of 95°C would require only around 0.015 milliseconds to fully denature the proteins. However, if the temperature was only 70°C, then the time needed increases to 35 ms.
The reason why there is such a big jump in the time required is due to the exponential nature of the denaturation process. In the Arrhenius equation above, we can see that the amount of protein damage, W, depends exponentially on the temperature, T. This means that a small increase in the protein temperature results in much faster denaturation.
I have also added the note that ‘bulk skin’ denatures in around 1 second when its temperature is around 60°C – this is often described as the “denaturation point” in skin. This interpretation is a misunderstanding, since skin will denature at any temperature above 55°C.
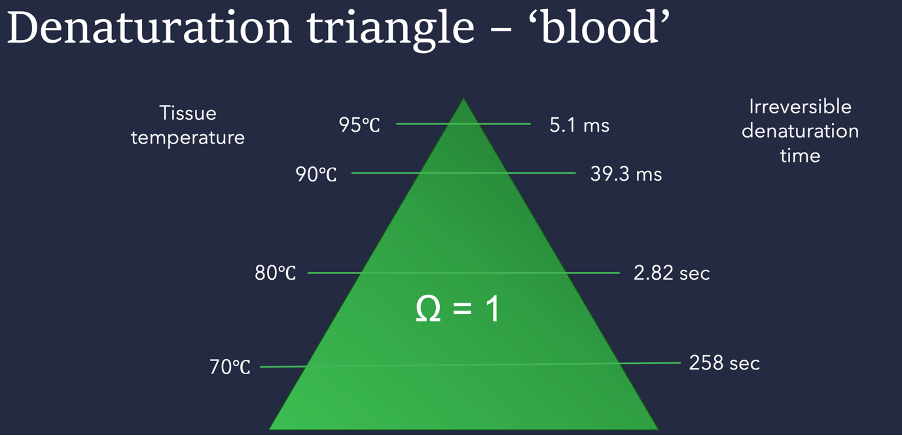
The above image shows the denaturation triangle for blood – note that the times required to fully denature are significantly longer than for ‘bulk skin’. This is because blood requires more much energy to begin the process of denaturation, but the proteins break down more quickly than in bulk skin.
Similar triangles may be constructed for any protein to show how their denaturation timings differ.
There are two interesting things to note here:
- The time needed to fully cook proteins is significantly shorter when higher temperatures are applied – this relates to the applied fluence at the skin surface in laser/IPL treatments. Higher fluences generate higher temperatures in the tissues;
- The tissues (proteins) all cook differently! Some denature more readily and faster than others. For example, when targeting blood vessels, the vessel wall will cook at a lower temperature than the blood.
Conclusion
In reality, we can cook tissues in a wide range of temperatures – they just take different times to reach the final goal. This gives us options. It explains why the ‘stamping’ and ‘SHR’ approaches both successfully kill hair follicles – different temperatures are attained for different time periods, but the same end result is possible.
Hope this helps,
Mike.


